Glaucoma treatment – a complete overview of all treatment and therapy methods
Effective treatment of glaucoma is essential to slow or stop the progressive loss of vision. It is important to understand that glaucoma cannot be cured as damage already done to the optic nerve is irreversible.
These methods of glaucoma treatment are available and are described in detail:
The main method of lowering intraocular pressure in glaucoma is the use of special eye drops. These preparations are applied once a day or several times a day, depending on the prescription. Regular check-ups at the ophthalmologist’s office are also of great importance and are usually performed every 1 to 3 months, depending on the progress of the disease.
In cases where drug treatment is not sufficiently effective or is not well tolerated, surgical procedures such as trabeculectomy or minimally invasive procedures may be considered.
In the event of an acute glaucoma attack, it is imperative to seek medical attention immediately to prevent damage to the optic nerve. In such emergencies, laser treatment can help to quickly reduce the congested intraocular pressure.
Therapy for glaucoma includes:
- Application of medication in the form of eye drops
- Laser interventions
- Surgical procedures
The main aim of these treatments is to lower the intraocular pressure to minimise the risk of vision damage and blindness.
The term glaucoma, refers to several eye diseases that all result in damage to the optic nerve. The most common glaucoma symptoms include:
- Increased intraocular pressure (IOP)
- Sensitivity to glare
- Loss of visual field
- Tunnel vision or reduced field of vision
- Reduced contrast vision
- Changes in colour vision
- Eye pain or headache
- Reddening of the eye
- Halos around light sources
The reduction of intraocular pressure is crucial to slow down or stop the gradual loss of vision in glaucoma. Nevertheless, it is important to emphasise that a cure for glaucoma is not possible, as existing damage to the optic nerve cannot be reversed.
In the following, we describe in full breadth and depth:
- The basic treatment options for each type of glaucoma
- The options for treating glaucoma in children
- The medicines used for glaucoma treatment
- The alternative treatment options
- New therapies for glaucoma treatment
- The different procedures for glaucoma surgery
- As a subgroup of surgeries, the different laser surgery procedures
- The glaucoma cure options
The Pseudoexfoliation Syndrome treatment (PEX glaucoma)
Treatment options for pseudoexfoliation syndrome focus on lowering intraocular pressure and thus reducing the risk of developing glaucoma. One of the first lines of treatment consists of eye drops designed to lower intraocular pressure.
This can be achieved by various types of medication that either reduce the production of aqueous humour or improve its outflow. If drug therapy is not sufficient, laser treatments such as selective laser trabeculoplasty can be considered to open the aqueous angle and improve the outflow of aqueous humour.
In advanced cases where the intraocular pressure cannot be reduced sufficiently, surgical intervention may be necessary. Surgical options include procedures such as trabeculectomy, in which a small hole is cut in the eyeball wall to allow the aqueous humour to drain, or canaloplasty, in which Schlemm’s canal is widened to reduce intraocular pressure.
Minimally invasive options such as the insertion of implants like the iStent or the XEN gel implant can also be considered to improve aqueous humour outflow.
In addition to these treatments, regular monitoring of intraocular pressure and ocular structure is crucial to monitor the progression of the disease and intervene in time. It is important to note that the treatment of pseudoexfoliation syndrome can be a lifelong effort to minimise the risk of glaucoma and associated visual damage.
The Angle-Closure Glaucoma (ACG) treatment
The treatment of angle-closure glaucoma includes various measures, depending on the acute or chronic form. In acute cases, which represent a medical emergency, immediate drug therapy is used to lower the intraocular pressure.
This is often supplemented by an iridotomy to open the chamber angle. In chronic cases, a laser iridotomy can also be performed to prevent the progression of glaucoma. Surgical removal of a cataract can also help to slow down chronic angle-closure glaucoma.
Principles of Ocular Hypertension Treatment
Ocular hypertension treatment includes various methods to control elevated intraocular pressure and prevent potential damage. A basic strategy involves regular monitoring and control of intraocular pressure.
If necessary, medications such as eye drops or systemic tablets are used to lower the pressure. These medications belong to different classes, including beta-blockers, prostaglandins, alpha-agonists, carbonic anhydrase inhibitors and cholinergics.
An alternative option to ocular hypertension treatment is laser trabeculoplasty to improve the outflow of aqueous humour, and in some cases surgery may be considered, particularly for planned cataract removal surgery. The selection of the optimal ocular hypertension treatment depends on individual factors and the severity of the disease.
Low-pressure Glaucoma treatment
The treatment of low tension glaucoma includes various methods to prevent damage to the optic nerve and to regulate blood pressure. Diagnostic methods such as pachymetry and ophthalmoscopy are crucial as the eye pressure is within the normal range.
Risk factors such as Flammer syndrome, sleep apnoea and metabolic syndrome can influence the development. Therapy is aimed at regulating blood pressure and changing lifestyle. This can be achieved through medication, lifestyle changes and regular medical check-ups.
Advanced cases may require eye drops, laser treatment or surgery. Early treatment improves the prognosis and continuous monitoring is crucial to prevent blindness.
The Pigment Dispersion Syndrome and Pigment Glaucoma Treatment
The treatment of pigment dispersion syndrome (PDS) and pigmentary glaucoma is crucial to prevent the progression of these eye diseases. In PDS, pigment cells detach from the back of the iris and can increase intraocular pressure, which can lead to pigment dispersion glaucoma if left untreated.
The cause often lies in the concave structure of the iris, which causes friction and releases pigment granules. Risk factors such as short-sightedness and genetic predisposition can favour the transition to glaucoma. Symptoms are often rare, but a sudden increase in intraocular pressure can lead to visual disturbances. Diagnosis requires a careful examination to rule out other diseases.
Treatment centres on controlling intraocular pressure through medication or surgical procedures such as laser treatment or trabeculectomy. Regular monitoring is crucial for the success of treatment and the long-term health of patients. Preventive measures include regular eye examinations, a healthy lifestyle and refraining from smoking.
The treatment of Narrow-Angle Glaucoma
The treatment of narrow-angle glaucoma is essential as it can lead to a sudden increase in intraocular pressure that requires urgent medical intervention. This can be caused by pupil dilation or structural changes in the eye.
Symptoms include severe headaches, nausea, and visual disturbances. Treatment options include iridectomy or YAG laser iridotomy to open the blocked chamber angle. Prophylactic measures, such as lens replacement, can also reduce the risk of a glaucoma attack.
Intermittent angle-closure glaucoma, a less aggressive form, has similar symptoms, but they are less pronounced and often only occur in certain situations. Diagnosis is based on the patient’s medical history and monitoring of intraocular pressure.
The treatment of Glaucoma in children
The treatment of juvenile glaucoma, a rare eye disease in children, requires an individualised approach. Diagnosis and treatment are complex and require specialised examinations to control the increased intraocular pressure and preserve vision.
Various surgical procedures such as trabeculotomy and cyclophotocoagulation are used to improve the drainage of the aqueous humour and reduce the pressure in the eye. Early intervention and regular monitoring are crucial to prevent visual damage and improve the quality of life of affected children.
The medicines used to treat Glaucoma
The aim of glaucoma treatment is to reduce intraocular pressure and prevent damage to the optic nerve. This is achieved using various medications, which can be administered topically as eye drops or systemically.
The most commonly used groups of medications include:
- Prostaglandin analogues
- Beta-blockers
- Alpha agonists
- Carbonic anhydrase inhibitors and
- Rho-kinase inhibitors.
These medications are often combined to achieve an optimal effect. In the event of a glaucoma attack, an acute emergency, specific medications such as pilocarpine solution are used to quickly reduce intraocular pressure.
The introduction of preservative-free preparations has expanded treatment options by reducing side effects for some patients.
Despite research into cannabis as a potential treatment option for glaucoma, questions remain about dosage and long-term effects.
The alternative Glaucoma treatment
These alternative approaches to glaucoma treatment include:
- Dietary changes
- Lifestyle changes
- Eye training
- Detoxification
- Meditation
- Acupuncture
- and other alternative medicine methods.
Diet plays an important role in regulating eye pressure, while exercise, stress management and eye care can also have an impact. Detoxification and elimination of waste products are other alternative approaches to glaucoma treatment, as are meditation and acupuncture.
Alternative practitioners take a holistic approach and can use various therapies such as anthroposophic medicine, acupuncture, nutritional therapy and reflexology on the foot to treat or prevent glaucoma.
New therapies for Glaucoma treatment
Progress in the treatment of glaucoma: New therapies bring hope for patients with optic nerve diseases. A promising gene therapy developed at Harvard Medical School has shown encouraging results in regenerating the optic nerve of glaucoma mice.
This ground-breaking method could represent a revolutionary treatment option for glaucoma patients and significantly improve their quality of life.
In addition, an innovative measurement method using an implantable chip promises to simplify the monitoring of intraocular pressure during cataract surgery, allowing patients to play an active role in controlling their health.
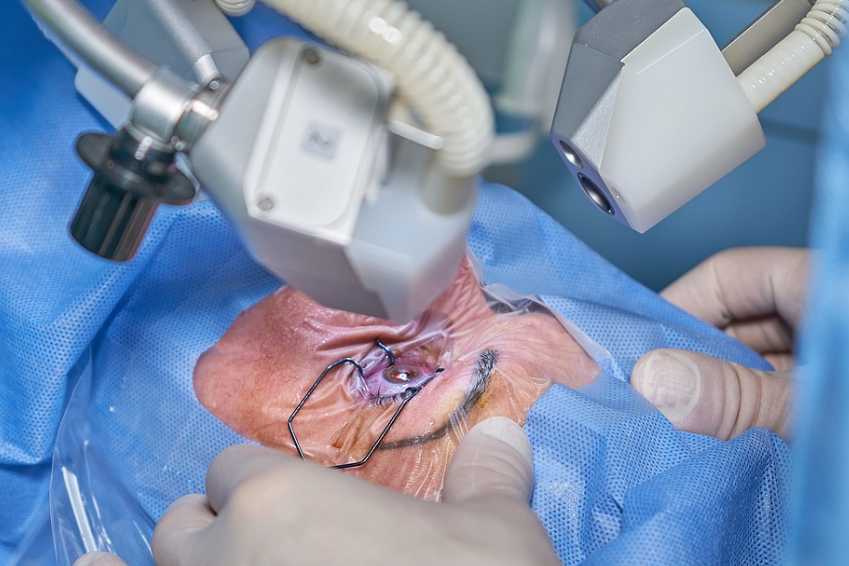
Glaucoma surgery
Glaucoma can be treated using various methods, including glaucoma surgery and laser treatments. These options are considered when medication alone is not sufficient or not well tolerated.
The three main types of glaucoma surgery and laser treatments include glaucoma surgery trabeculectomy, laser trabeculotomy and laser iridectomy. Each method has its own advantages, disadvantages and risks that should be carefully considered before making a decision.
The choice of the appropriate treatment depends on individual factors such as the condition of the eye and the patient’s preferences.
Glaucoma Laser surgery – Selective Laser Trabeculoplasty (SLT)
Selective laser trabeculoplasty (SLT) is a method of treating glaucoma in which the outflow channel in the angle of the eye is widened by laser treatment in order to create new outflow possibilities for the aqueous humour.
This treatment can help to reduce the strain on the optic nerve. SLT is particularly suitable for patients with open-angle glaucoma and offers an alternative for those who have difficulty with glaucoma drops.
The chances of curing Glaucoma and an outlook
Although glaucoma cannot be cured, there are promising developments in research that could potentially lead to a cure. New medications, genetic and cellular regeneration therapies as well as advances in cataract surgery and innovative stents offer hope for more effective treatment options.
Future technologies such as contact lens-based drug delivery systems and implantable drug pumps show promising ways in which glaucoma could be treated more effectively to improve the chances of a cure.
Augenakupunktur Noll treats degenerative eye diseases
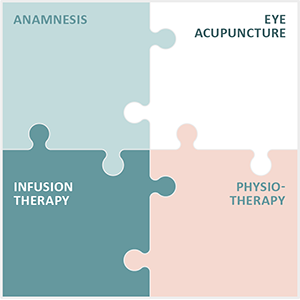
The practice Augenakupunktur Noll specialises in the treatment of degenerative eye diseases. To this end, Michaela Noll has developed and continuously refined the Integrated Eye Therapy according to Noll. It consists of 4 elements:
- A comprehensive anamnesis
- Eye acupuncture according to Prof. Boel
- Individual infusions
- Special physiotherapy
Eye acupuncture according to Prof. John Boel is at the centre of the therapy. Based on the anamnesis, a treatment concept is developed for each patient that is customised to their illness or impairment.
In its 13 years of operation, the practice has built up an international patient base and prides itself on the positive feedback it receives from patients after their treatments.